The Latest Advancements in the Treatment of Age-Related Macular Degeneration
&srotate=0)
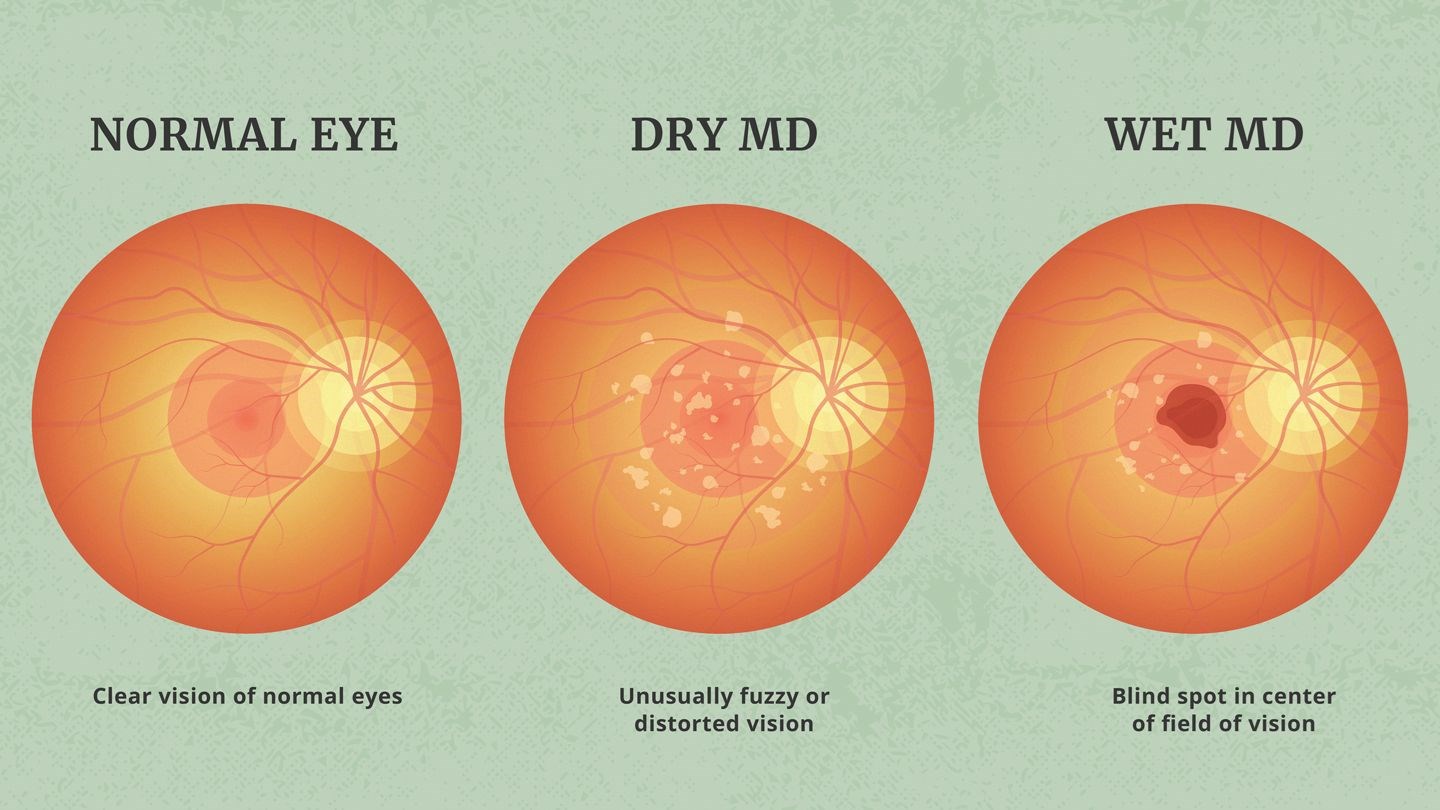
Dry AMD vs. Wet AMD vs. Geographic Atrophy
Dry AMD, also known as non-neovascular AMD, is characterized by the presence of drusen - small, yellow deposits that accumulate in the macula, the central part of the retina. Drusen can lead to a gradual breakdown of light-sensitive cells in the macula, resulting in a loss of central vision. Dry AMD typically progresses slowly and is the most common form of AMD.
Wet AMD, on the other hand, is less common but more severe. It occurs when abnormal blood vessels grow beneath the macula and leak blood and fluid, causing rapid and severe damage to central vision. Symptoms of wet AMD often include distorted or wavy vision, as well as the appearance of blind spots.
Geographic Atrophy (GA) is a severe form of dry AMD, characterized by the gradual loss of cells in the macula. This results in the formation of well-defined, atrophic areas in the retina, hence the term “geographic.” GA can lead to a significant and irreversible loss of central vision.
New Advances
Exciting developments in AMD treatment have recently emerged, offering hope to patients facing vision loss. One such breakthrough is the FDA approval of Izervay (avacincaptad pegol) for the treatment of geographic atrophy secondary to AMD. Izervay is administered via intravitreal injection, delivering the medication to the back of the eye.
Izervay is a complement C5 inhibitor, targeting the overactivity of the complement system and the C5 protein. These elements are believed to contribute to the scarring and vision loss associated with geographic atrophy. Developed by Iveric Bio, Izervay has shown promise in slowing the progression of GA by addressing the source of retinal cell death and potentially preserving the benefits of the complement system.
The approval of Izervay was based on rigorous clinical trials, known as the GATHER1 and GATHER2 phase 3 trials. These studies evaluated the safety and efficacy of monthly 2 mg intravitreal injections of Izervay. Over a 12-month period, Izervay demonstrated a statistically significant reduction in the rate of geographic atrophy growth. Notably, this slowing of disease progression was observed as early as six months into treatment, with up to a 35% reduction in the first year.
Precautions
While these advances offer new hope, it’s important to note potential precautions. Patients receiving Izervay may experience common adverse reactions such as bleeding beneath the clear lining of the eye, increased intraocular pressure, and blurred vision. Close monitoring and consultation with a qualified ophthalmologist, like Dr. Ticho, are crucial to managing these potential side effects and optimizing treatment outcomes.
Key Points
Age-related macular degeneration (AMD) encompasses dry AMD, wet AMD, and geographic atrophy (GA), each with distinct characteristics and treatment approaches.
Izervay, an FDA-approved complement C5 inhibitor, shows promise in slowing the progression of geographic atrophy secondary to AMD, preserving central vision.
The approval of Izervay is based on the GATHER1 and GATHER2 phase 3 clinical trials, demonstrating significant reductions in GA growth rates.
Patients considering Izervay should be aware of potential adverse reactions and should consult with their ophthalmologist for comprehensive care.
Ticho Eye Associates, with practice locations in Chicago Ridge, Tinley Park, and Munster, Indiana, is dedicated to providing state-of-the-art eye care, including fundus autofluorescence testing to detect macular degeneration at its earliest stages.
In conclusion, the evolving landscape of AMD treatment offers new hope for patients dealing with these sight-threatening conditions. The doctors at Ticho Eye Associates, with practice locations in Chicago Ridge, Tinley Park, and Munster, Indiana, provide patients and healthcare providers with a deeper understanding of AMD and the latest advancements in eye care. For consultations and appointments, please contact Ticho Eye Associates at 708-873-0088, where our dedicated team is committed to preserving and enhancing your vision.